Plants in pots even work in garden especially when you give the pots and plants in them, enough room to become a nice focal point or a nice addition to plants that are in the garden. A focal point can be one plant in a pot against an otherwise barren wall, where the colours of the plant(s) contrast with the colour of the wall. Complementary pot plants can be like you extend or accentuate the colour scheme of that particular garden area by putting a tropical or unusually shaped plant in a complementary colour.
One of the most evident mistakes is to put plants of different groups in the same pot. You have to make sure, the plants you put in one pot, have to have same nutritional and watering needs.
Groups of plants that be put together, in the same pot are:
Each plant group has its own needs as far as type of soil, drainage, watering, location (sunny or shady,…) … are concerned. If you want to do more complicated of plants within the same group, it is important to realize that either you stick to similar colours of try to create a display with contrasting colours. The colour wheel shows contrasting colours on the opposite side.
Pots come in a variety of materials and size. Here are some basic rules to choose a pot. While you may not have a wall of pots to choose from, it's a good idea to keep a selection of various shapes, sizes, and materials on hand.
Know your plants. Some pot materials allow water and air to pass through them, promoting drainage in the mix but also accelerating water loss. Most plants benefit from the presence of a drainage hole; if lacking one, most containers can be drilled to make one.
Know your materials.
Storage conditions of belgian endive, chicory, witloof, whitloof?
This is a traditional Belgian recipe !
Ingredients (For 4 persons)
Boil in a big cooking pot water with salt. Put the rinsed (entire) pieces of endive in the boiling water for 12 minutes. Scoop them out and let them drain. Keep 2 dl of the boiling liquid.
Melt the butter in saucepan and add flower. Keep stiring during 2 minutes while whisking. Add 1 dl of the boiling liquid en let cook in to get the sauce thicker. Keep stirring. Add half of the milk, let it boil up and then add the rest of the boiling water, the milk and the white wine.
Let the sauce simmer for 5 minutes. Stir ¾ of the grated cheese and the musterd in the sauce till you get a thick smooth sauce. Take the pan off the fire and add the eggjoke and add salt and pepper to your taste. Preheat the oven on 180 °c. Remove the foot of the endives, cut them in half on their length and wrap the ham around them. Put them in a big oven dish and add a bit of pepper. Stir the cheese sauce once more and poor it over the endive-ham rolls. Cover this with the rest of the cheese and add nutmeg and put it in the oven for 20 minutes on 180 °C. Then turn up the heat of the oven till 200 °C and let it bake for another 10 minutes or till the cheese sauce has a nice golden brown colour.
Have a nice meal!
 |
| Hippeastrum Minerva |
Common name: Amaryllis. A label given by sellers and nurseries, and care should be taken not to confuse it with Amaryllis belladonna.
Images: see below
Origin: South America, Central America and the Carribean
Type: perennial
Plant description: Long, rather narrow leaves, six-petaled flower, which can grow up to 6 inches wide.The plant is often mistaken for a lily
Size: stem and leaves grow to up to 22 inches.
Light: Amaryllis loves sun, and you should find it the most well-lit place possible
Temperature: 20-30 degrees centigrade
Soil: nutritious potting compost; well-drained soil.
Watering: water sparingly until the bud and leaves appear, then increase waterings
Fertilizer: use half the recommended rate of any water soluble fertilizer.
Two or three applications of fertilizer during the growing season (March through September).
 | |
| Hippeastrum cybister hybrid |
Propagation: Amaryllis can be propagated by seeds, offsets (bulblets) or cuttage. The best way to propagate Amaryllis is by separating the small bulbs which grow on the ''mother'' bulb. The bulblets can be divided during fall, when the leaves begin to turn yellow. Offsets should bloom within approximately three years.
An interesting experiment is to propagate Amaryllis from seeds. When the Amaryllis is in bloom, take a cotton swab and run it across the stamen. For pollination leave only one flower per stem. Then brush the pollen on to the tip of the pistil.
After the flower starts fading, a bulge will grow at the base of the bloom. Riping will last for about 2 months. When ripe, the pod will eventually burst open. Collect the seeds. They need to be sowed soon, otherwise they will lose viability. Not all of them will be viable, but you can collect about 20-40 viable seeds.
Sow the seeds in soilless substrate Keep growing medium moist, warm and in light. They will sprout randomly after at least three weeks. Baby Amaryllis should be transplanted only when they are big enough to be handled. It may take up to three years until you will have the first bloom and the plants resulted will not be identical to the mother plant.
Pests and diseases: the narcissus bulb fly, red bloch, a fungus disease
Extra tips:
When buying the bulb, keep in mind that the bigger the bulb, the more flowers you will have
It is not necessary to separate the bulbs every year, but doing so will encourage uniform flowering and larger flowers.
Remove the dead blooms before the plant produces the seeds. Otherwise, flowering during the next season will be greatly reduced.
Carefully inspect the bulbs before buying, in order to prevent diseases.
Fertilizers (or fertilisers) are substances that supply plant nutrients or amend soil fertility. They are the most effective means of increasing crop production and of improving the quality of food and fodder. Fertilizers are used in order to supplement the natural nutrient supply in the soil, especially to correct the (yield-limiting) minimum factor.
Fertilizers are soil amendments applied to promote plant growth; the main nutrients present in fertilizer are nitrogen, phosphorus, and potassium (the 'macronutrients') and other nutrients ('micronutrients') are added in smaller amounts. Fertilizers are usually directly applied to soil, and also sprayed on leaves ('foliar feeding').
Organic fertilizers or and some mined inorganic fertilizers have been used for many centuries, whereas chemically synthesized inorganic fertilizers were only widely developed during the industrial revolution. Increased understanding and use of fertilizers were important parts of the pre-industrial British Agricultural Revolution and the industrial green revolution of the 20th century. Inorganic fertilizer use has also significantly supported global population growth — it has been estimated that almost half the people on the Earth are currently fed as a result of artificial nitrogen fertilizer use.[1]
Fertilizers typically provide, in varying proportions:
Critical values are still quite useful and are frequently referred to when interpreting a plant analysis result. A brief discussion of the known critical values for the elements included in a plant analysis is given below:
Nitrogen (N)
The critical level of N in many plants is around 3 percent. For several crops, when the N level in leaves drops below 2.75 percent, N deficiency symptoms appear and yield and quality decline. The primary exceptions are for the very young plants when the critical level may be 4 percent or more, and for leguminous plants, such as soybeans, peanuts, alfalfa, etc., where the critical N percentage is 3 to 4.25 percent. For some tree fruits and ornamentals, N levels may be as low as 2 percent before deficiency occurs. Deficiencies as well as excesses can be a problem. Nitrogen leaf levels in some varieties of pecans exceeding 3.50 percent may result in early defoliation. Nitrogen leaf levels greater than 4.50 to 5 percent retard fruit set in greenhouse tomato. High N levels (>3.50 percent) in forage crops such as fescue is thought to be related to the incidence of grass tetany.
Small changes in N content for some crops can result in large effects on yield, plant growth, and the quality of forage and fruit. Therefore, it is important that the N level be maintained within the prescribed limits of the sufficiency range by the proper use of N fertilizer.
Phosphorus (P)
The P requirement of plants varies considerably. Tree crops have relatively low P requirements with the critical values ranging from 0.12 to 0.15 percent. Grasses have higher P requirements with critical values ranging from 0.20 to 0.25 percent. Legumes and some vegetable crops have relatively higher P requirements with critical values being 0.25 to 0.30 percent or slightly higher. Most plants grow to the extent to maintain a near constant P level within the plant. When a P deficiency occurs, it is usually due to a severe inadequacy of P in the soil solution, or in some cases it may be due to a restricted root system as a result of cool-moist growing conditions. Phosphorus deficiencies normally occur early in the growth cycle of the plant when the P requirement is high. The P content of plants is initially high and declines with age. Since P is a fairly mobile element in plants, deficiencies generally occur on older tissue.
The excess range of P is not clearly known. The P level in young plants can be very high such as 0.50 to 1.00 percent, but these high levels may reflect actual need. In some instances, high P plant levels may cause imbalances and deficiencies of other elements, such as Zn, Cu, Fe, etc. Plant P can be maintained within the sufficiency range by proper P fertilization and the maintenance of the soil P level within the medium to high soil test range.
Potassium (K)
The K requirement of plants varies widely depending on plant species. The tree crops such as pecans, peaches, apples, etc., have relatively low K requirements. The critical value for K in tree leaves ranges from 0.75 to 1.25 percent. For grasses, the K requirement is higher with the critical value in leaves ranging from 1.20 to 2.00 percent. For legumes, the critical value for K generally ranges from 1.75 to 2.00 percent. The K level in a plant can change quickly as K is quite mobile and moves readily within the plant. Potassium can be easily leached from growing plants by rain to be reabsorbed through the roots. Because of K mobility, both in the plant and soil, deficiency symptoms can develop quickly. Deficiencies frequently occur during both the early and latter stages of growth, particularly during fruiting. Young plants may contain 3.00 to 5.00 percent K, although the actual requirement may not be that high. Because it is mobile in the plant, K deficiency symptoms appear in the older plant tissue first. The K concentration in the plant decreases with age. Potassium balance in plants is important. The K/(Ca+Mg) and K/N balances must be maintained at a proper level to avoid deficiencies of Mg in the first instance and K in the second. High K can induce Mg deficiency in most plant and tree crops. Plants which are Mg deficient may have high K and Ca contents as the plant tends to maintain a constant cation concentration. As a result of these balance phenomena, heavy applications of K or N fertilizer, respectively, can induce a Mg or K deficiency. Under Georgia soil conditions, K deficiency is difficult to induce unless the K soil test level is low and the soil is heavily limed or fertilized with large quantities of N. The K to N balance is becoming increasingly important in pecans. As the N level in tree leaves increase, the K level must also be increased to maintain the proper balance and prevent K deficiency from occurring. Plant K can be maintained within the sufficiency range by proper K fertilization and the maintenance of the soil K level within the medium to high soil test range.
Magnesium (Mg)
Magnesium deficiency occurs in many plants when the leaf level is less than 0.10 to 0.15 percent. Small grains may exhibit deficiency symptoms when the Mg level is less than 0.10 percent. When corn is less than 12 inches in height, magnesium deficiency may occur when the Mg level is below 0.15 percent. However, as corn matures, deficiencies may not be evident until the Mg level is less than 0.13 percent. For legumes such as peanuts and soybeans, the critical level is 0.25 to 0.30 percent. The critical level for cotton and pecans is 0.30 percent. Several vegetable crops such as tomato, turnips, and collards have a high Mg requirement with the critical level near 0.40 percent Mg. Magnesium is a fairly mobile element in the plant, therefore, deficiency symptoms occur in the older plant tissue. The Mg concentration in the plant tends to increase with age.
Magnesium deficiencies can be induced by excessive K and NH4-N fertilization. When the soil pH is less than 5.4, Mg availability and uptake by plants is greatly reduced. The usual cause for Mg deficiency in Georgia is generally low soil pH and/or low soil Mg. Depending on the soil conditions, the effect of K and NH4-N fertilization can vary depending on the soil pH and level of soil Mg. Continued liming with only calcitic lime will result in a Mg deficiency. Adequate soil Mg can generally be maintained by liming with dolomitic limestone to keep the soil pH between 6.0 and 6.5. Supplemental applications of fertilizer Mg may be needed in some cases to supply some of the Mg crop requirement.
Sulfur (S)
It has been generally thought that the S requirement of plants was comparable to that of P. This has not proven to be so. The S requirement for grasses is quite low, the critical value being around 0.10 percent. Sulfur deficiencies in corn do not generally occur until the S level is less than 0.13 percent in the leaves. Under Georgia conditions, legumes, cotton, tobacco, and tomatoes have a critical S level of about 0.20 to 0.25 percent. The S critical level for crops such as cabbage, spinach, turnips, and collards is around 0.30 percent. However, additional research in this area should aid in pinpointing the critical level for these crops. There is a critical N to S percentage ratio which should be maintained. As suggested by Reneau (1983) the N:S ratio may be a better indicator of the S status of corn than the S concentration. For crops such as corn, this ratio should not exceed 18:1 if S deficiency is to be avoided. Stewart and Porter (1969) suggested that a N:S ratio above 16:1 indicates a lack of S may be limiting protein formation. A ratio of 20:1 or greater indicates that S is severely deficient. For optimum corn grain yields, the N:S ratio should be maintained between 10:1 to 15:1 (M. E. Sumner, personal communication). The optimum N:S ratio for Coastal bermudagrass ranges from approximately 9:1 to 12:1 (Martin and Matocho, 1973). Maintaining the N:S ratio within the range for optimum production of Coastal also provides the N:S ratio that is about optimum (10:1 to 15:1) for ruminant nutrition (Allaway and Thompson, 1966). Sulfur deficiencies occur primarily on the very sandy soils of South Georgia and when low S containing fertilizers are used over several years. Sulfur deficiencies tend to occur early in the plant growth cycle. The proper S level can be maintained in the plant by providing a S source near the germinating seed or by adding S with sidedress and topdress N applications particularly in sandy soils. Most Georgia subsoils contain sizeable quantities of S. Provided the pH is not too low when roots enter the subsoil, sufficient S will generally be available to satisfy the crop requirement.
Since S is not a mobile element in the plant, deficiency symptoms tend to first appear in the upper or newly emerging leaf tissue.
Calcium (Ca)
The Ca requirement for plants varies widely with grasses having the lowest requirement, legumes intermediate, and fruit crops and cotton the highest. Calcium levels from 0.20 to 0.25 percent are quite adequate for pasture grasses and corn. Soybean has a critical Ca concentration in the mature leaves of 0.50 percent, while the level for peanuts is 1.25 percent. Apple leaves should contain about 1 percent Ca and peach leaves 1.25 percent. Greenhouse tomato has a critical concentration for leaves of about 1 percent. Of the crops grown in Georgia, cotton probably has the highest critical Ca concentration at 2 percent for leaves.
Calcium deficiencies are not unusual, although the crops where Ca is particularly important are the fruit crops, such as apples, peaches, and tomato. Calcium deficiency will significantly affect fruit quality. Brown rots, easy bruising of fruit, and blossom-end rot of tomato are frequently associated with inadequate Ca. Pod-rot in peanuts is also a Ca deficiency. These deficiencies are not easily "uncovered" by leaf analysis. When Ca deficiency is severe, newly emerging tissue is affected. The margins of the leaves tend to stick together, giving a ragged edge to new leaves. Older leaves will show a browning of the margins. Since Ca is not a mobile element, deficiencies occur in the newer tissues. The Ca level in plants tends to increase with the age of the plant.
There is increasing evidence that Ca is more like a micronutrient, as the critical concentration may be in the parts per million range. Several plant physiologists have grown plants successfully at low Ca levels in artificial growth media. In these experiments, the balance of Ca with the other essential elements such as Mg, Cu, Fe, B, and Mn was critical. Calcium was found to be sufficient with plant and leaf concentrations between 600 ppm to 1000 ppm. It is known that relatively little Ca is in a soluble form in many plants. Crystals of calcium oxalate have been observed in the leaves of most fruit trees as well as some field crops which are thought to have high Ca requirements. Therefore, the sufficiency of Ca in such plants may be related to the soluble fraction in the leaves rather than the total. Unfortunately at this time, all of the current literature related to Ca and its sufficiency concentration are based on total Ca contents of sampled plant parts. No doubt there is need to change the method of analysis for Ca to determine the soluble Ca content and relate this to sufficiency range standards.
Manganese (Mn)
Manganese deficiency normally occurs when the leaf tissue concentration is less than about 15 ppm. Depending upon the crop, ample but not excessive concentrations of Mn may range from 15 to over 1,000 ppm. Although there is limited data to delineate when toxicity occurs, leaf levels in excess of several hundred ppm are probably toxic to many plants. Plants which are sensitive to Mn deficiency are equally sensitive to excessive Mn. Growth of soybeans, which are particularly sensitive to Mn deficiency, is reduced when leaf Mn levels approach 200 ppm (Ohki, 1976). Several plant species have higher Mn critical levels. For example, the critical Mn level for alfalfa is about 25 ppm.
Some plants can tolerate extremely high Mn levels without detrimental effects. Pecan leaves may contain up to 1000 ppm Mn with seemingly no adverse effect. Similarly, cotton and peanuts will accumulate Mn up to 500 ppm without apparent toxicity. However, a high Mn level in plants is a sign of low soil pH, and is frequently associated with Mg deficiency. When the Mn concentration in peach leaves exceeds 150 ppm, this is generally a good indication that the soil pH is low according to George Cummings.
The Mn level in plants is usually quite high at the initial period of growth. It decreases rather rapidly and then levels off to remain fairly constant during most of the season. Since Mn is not a mobile element, deficiency symptoms will occur in the newer leaves or upper portion of the plant.
Iron (Fe)
Iron analyses are probably invalid unless the leaf tissue has been washed in dilute acid or detergent solutions. Therefore, for unwashed leaves, iron analyses are of no real value. When soil contamination is suspected, usually Al is also high.
The Fe content in a plant can vary considerably. In general, when the Fe concentration in leaves is 50 ppm or less, deficiency is likely to occur. The grasses and corn have a lower Fe requirement, the critical level being 20 ppm. Iron toxicity has not been reported for any field crops growing under natural conditions in Georgia. The only Fe sensitive field crops would be pecans and soybeans, with possible deficiency occurring only on soils with pH's at 7.0 or above. Iron deficiency is common in Centipede grass and azaleas, particularly when grown in soils with pH's above 6.0
Iron deficiency is very difficult to correct in some crops. The application of some forms of Fe to the soil is not practical. Foliar applications of Fe have been found to be effective in correcting Fe deficiencies in plants such as turf grasses. However, on crops such as pecans, foliar applications for correction of low Fe levels have been erratic.
Since Fe is an immobile element in plants, Fe deficiencies appear in the new tissue or upper portion of the plant. Iron deficiency symptoms may appear early in the growth of the plant only to disappear in several days or weeks. The Fe level in the plant usually remains fairly constant during the growing season.
Boron (B)
Boron requirements vary considerably among crops. The optimum range in leaf tissue of most crops is from 20 to 100 ppm. Some crops are particularly sensitive to B and can be injured when the leaf B level is too high. For example, B levels in excess of 50 ppm have been associated with B toxicity in peaches. The B critical level for corn is about 4 ppm, while alfalfa, cotton, peanut,and soybeans have critical levels of 20 ppm. Corn, having a fairly low B requirement, is also sensitive to excess B. Toxicities may occur when the B level in young corn leaf tissue exceeds 25 ppm. Members of the Papilionaceae and Cruciferae have fairly high B requirements with critical levels being about 25 to 30 ppm B in the leaf tissue. Those plants which have fairly high B requirements are also ones with fairly good tolerance to excessive B. Boron is not a very mobile element and deficiency symptoms occur in the newly emerging tissue. The B concentration in leaves remains constant during the growth cycle. Boron deficiencies result in various physiological diseases in plants, such as "hollow heart" in peanuts, a fairly common disorder occurring in Georgia peanut fields.
Copper (Cu)
The normal range of Cu in many plants is fairly narrow, ranging from 5 to 20 ppm. When the Cu concentration in plants is less than 3 ppm in the dry matter, deficiencies are likely to occur. When Cu levels exceed 20 ppm in mature leaves, toxicities may occur. There is some variation in the critical values for various plant species; however, most critical values have been determined to be somewhere between 3 to 10 ppm for most crops. The Cu level in leaves tends to remain constant during the growing season.
Copper deficiency symptoms often depend on plant species or variety and the stage of deficiency. In the early stages of deficiency, symptoms are generally reduced growth. In the moderate to acute stages of deficiency on crops such as wheat, terminal or new leaves are pale green, lack turgor, and become rolled and yellowed; older leaves become limp and bent at the ligule. The leaves die and dry to a bleached gray (Reuther and Labanauskas, 1966).
Zinc (Zn)
The normal range of Zn in most plants is between 20 to 100 ppm. Zinc deficiencies occur in a wide variety of plants when the leaf level drops below 15 ppm. The critical Zn value for apple is about 14 ppm with the first symptom of the deficiency being small fruit size. Zinc deficiency in pecans occurs when the Zn leaf level is 30 ppm or less.
In order to avoid Zn deficiency, Zn levels in most crops should be maintained at 20 ppm or better, except for pecans when 50 ppm Zn is the desired minimum.
Zinc toxicity is an uncommon problem and does not generally occur until the Zn level exceeds 200 ppm. However, in crops such as peanuts, Zn toxicity has been reported in Georgia when tissue levels reach 220 ppm (Keisling and others, 1977). More recently (Parker and Walker, 1986) reported that Zn levels up to 287 ppm did not adversely affect peanut yields nor show any of the symptoms associated with Zn toxicity. However, the author has observed plants exhibiting Zn toxicity symptoms, described by Keisling and others (1977), with Zn concentrations of 117 ppm. Apparently, there are other plant growth factors or nutrient relationships in addition to just the Zn concentration that affect the manifestation of Zn toxicity. One such relationship appears to be the Ca:Zn ratio in the tissue. Upon evaluating unpublished data of Parker in which the Zn concentration in tissue varied from 50 to 302 ppm, and Zn concentrations could not be related to Zn toxicity, the author noted that when the Ca:Zn ratio was less than approximately 45 to 50:1 Zn toxicity symptoms were evident. However, when the ratio was greater, where the Zn concentration was 302 ppm, no toxicity symptoms were detected. Continued research in this area should elucidate the nature of this relationship. Excessive Zn also interferes with the normal function of Fe in plants giving rise to symptoms similar to Fe deficiency.
Zinc is not a very mobile element in plants, and deficiency symptoms occur in the newly emerging leaves. Stunting is a frequent symptom associated with Zn deficiency. Zn concentration in leaves remains fairly constant with a fairly rapid increase at the end of the growth cycle.
Aluminum (Al)
Aluminum is not considered a plant nutrient; therefore, it is not required by plants. However, its presence in plants can affect the normal function of some other elements. As with Fe, probably no accurate measure of the Al status of the plant can be obtained unless the tissue is free from dust and soil contamination. High Al in plants is usually an indication of very low soil pH or poor soil aeration due to compaction or flooding. Aluminum levels in excess of 400 ppm in young tissue or 200 ppm in mature plants and leaves are undesirable.
Molybdenum (Mo)
Molybdenum deficiencies occur in many plants when the plant concentration is less than 0.10 ppm. Toxicity levels in plants have not been established. Molybdenum is quite toxic to animals if the forage being consumed contains more than 15 ppm Mo. The Mo requirement of legumes is higher than that of other plants since Mo is essential for the fixation of atmospheric N by the symbiotic bacteria. For the non-legumes, Mo is probably not needed if all the N requirement is supplied by the ammonium form. Molybdenum is essential for the conversion of nitrates to ammonium in the plant. In Georgia, Mo application has been found beneficial for alfalfa, particularly when the soil pH is low. The need for Mo on soybeans has also been confirmed in Georgia, particularly on the heavier Piedmont, Mountain, and Limestone Valley soils which are low (approximately 5.5 or less) in pH. Significant responses to Mo application have not been consistent on Coastal Plain soils. [2]
Citations
[1] http://en.wikipedia.org/wiki/Fertilizer#Inorganic_fertilizer_.28synthetic_fertilizer.29
[2] http://aesl.ces.uga.edu/publications/plant/Nutrient.htm#N
Ingredients for your soil mixes will be shown below.
Leaf mold
It is obtained from rotting leaves and it has a high cationic exchange capacity, good mineral content but it is missing macronutrients (Nitrogen, Phosphorus and Potassium). It has a lower pH and may contain agents for diseases. It should always be sterilized. Coarse texture is best used in rooting media or potting mixes.
 |
| Well rotten compost |
Compost
Compost results after the decaying organic material under the influence of fungi and bacteria. Because of the existence of microorganism it may protect your plants against diseases. It has a high cationic exchange capacity and a good balance between macronutrients and minerals.
 |
| Fine washed river sand |
It is used for a better drainage of your soil because the sand grains have different sizes and the water is drained quicker leaving air spaces in between. It is inert and it does not contain any nutrients. Use always washed sand and never use sand from the beach because it contains killing amounts of salt.
 |
| Grit |
Use always washed grit or coarse sand to improve drastically the aeration and drainage of your mixes. It mostly used for the rooting of cacti and other desert plants that require a more open medium.
 |
| A brick of dehydrated cocopeat |
Usually are sold in forms of dehydrated bricks that need to be rehydrated before use. It is derived from composted coconut shells and it is good replacement for less fine sphagnum moss in soilless substrates.
 |
| Perlite |
It is resulted from expanded volcanic rocks. It retains the water good and because it is inert it also drains water quickly. It is used in sterile mixes to improve drainage and aeration.
 |
| Potting soil |
Use soil of the best quality for seed germination and plant propagation. The soil must free of any other seeds, insects eggs. This soil is used for rich soil mixes.
Coir
Coir is a fibre resulted from coconut husks used as peat substitute. It dries out less quickly that peat but
requires more feeding. It is a good base for soilless mixes.
 | |
| Peat |
Is stable, long lasting and well aerated and retains the water well, but low in nutrients. It is difficult to re-humidify once it has gone dry. It is used for lightweight mixes intended for short use.
 |
| Dried and groud moss |
 |
| Sphagnum moss |
Do not use coarse sphagnum moss in propagation for this is intended mostly as an orchid medium. Use finely ground sphagnum moss for soil mixes intended for seed germination and semi-coarse for cuttings media.
 |
| Vermiculite (magnified photo) |
It is expanded and air-blown mica. It contains traces of Magnesium and has high cationic exchange capacity. Acts similarly as perlite but holds more water and less air. It supports drainage and aeration. It comes in various degress of grinding. Some of the finest are used on the top part of the substrate as a physical barrier against harmful bacteria and fungi.
Fine bark
Fine bark or chipped barked is used as peat substitute or for free draining acidic mixtures, especially for orchids, palms and other indoor plants.
Recipes for mixes
Making your own mix has the advantage of knowing what your mix contains. Accordingly, you can realize the ideal medium for a specific type of propagation technique or plant. You can find in the shops ready-made soil mixes generically Seeding and Cutting Soil Mix. When you mix your substrate, hygiene is very important. Use only clean tools and sterilized soil.
How to sterilize soil?
If you are planning to use garden soil in your mixes it is crucial that you sterilize it before incorporating it. Sterilization will kill possible harmful organisms that could affect seedling and cuttings. To do this the soil must sifted to remove clumps, stones and any other residue, but also to obtain a finer structure. Sterilizing garden soil for commercial use is done in special units. These are expensive and unnecessary for home use. An oven or a microwave would do just fine.
For soil sterilization in an oven you will need a deep baking tray, of about 8 to 10 cm. Bake the soil for about 30 minutes on 200 degrees C. You should know that unpleasant badly-smelling vapours may be released.
In order to sterilize soil in the microwave you need a roasted bag, resistant to high temperatures. After soil is inserted seal the top of the bag to avoid the contamination of the microwave. However, make a few holes and place in the oven for 10 minutes on maximum power.
Observation: some of the substrate mixes cited below, require some addition of non-biological nutrients. You may avoid using those mineral nutrients by excluding them from the mixes. Later on we will publish some ways in which you can obtain your own biological fertilizers.
Substrate recipes for potting mixes, rooting media and seeding mixes.
| Rich potting mix | Soilless potting mix | Soil based seeding mix |
| 2 parts compost 1 part potting soil 1 part sand 1 part vermiculite 1 part perlite | 3 parts peat or substitute 1 part sand or perlite For each 36 liters add 14 g ammonium nitrate 28 g potassium nitrate 45 g superphosfate 85 g ground limestone 85 g dolomitic limestone | 2 parts potting soil 1 part peat or substitute 1 part sand |
| 7 parts potting soil 3 parts peat or substitute 2 parts sand For every 36 litres add 113g general purpose slow release fertilizer and 21 g limestone | 3 parts peat or substitute 1 part sand or perlite For each 36 liters add 14 g ammonium nitrate 28 g potassium nitrate 45 g superphosfate By avoiding the use of limestone you will obtain an acidic composition | 2 parts potting soil 1 part peat or substitute 1 part sand For each 36 litres you may also add 42g superphosfate 21 g ground limestone |
| 7 parts acidic potting soil 3 parts peat or substitute 2 parts sand For every 36 litres add 113g general purpose slow release fertilizer This potting mix is used for acidophile plants. | 2 parts potting soil 1 part peat or substitute 1 part sand For each 36 litres you may also add 42g superphosfate |
| Soilless seeding mix | Soilless rooting media | Sterile substrate |
| 3 parts peat or substitute 1 part fine bark 1 part perlite For each 36 litres add 36 g trams of slow release fertilizer | 1 part peat or substitute 1 part sand (or perlite or vermiculite) | 1 part finely ground Sphagnum Moss 1 part perlite |
| 1 part perlite 1 part vermiculite 2 parts finely ground sphagnum moss | 1 part peat 1 part fine bark To each 36 litres add 36 g of slow release fertilizer | 1 part cocopeat 1 part perlite |
| 1 part peat 1 part fine bark 1 part perlite To each 36 litres add 36 g of slow release fertilizer |
General , Germination , Propagation , Soil
People who like Belgian endive like it so much they grow it twice. In fact, they have no choice. Endive, perhaps the most famous member of the chicory family, is grown in two stages, once for the roots, and a second time for its yellow and white leaves. It is a particularly welcome member of the family in that it can produce crunchy salads throughout the entire winter if you grow enough roots.
 Pack the roots upright in a bucket or pail and fill around them with sand, if you have it, or loose sandy soil if you don't. Regular soil or peat can also be used, but it is difficult to use for filling in the gaps. Cover and store pails in the coolest location available. If you don't have a dark enough room, just use 2 flowerpots, one to hold the roots and another to put on top, to provide you with a dark growing area. Three weeks before you want to enjoy your first endive feast, move the bucket to a 50-60 degree (10-15 celsius) location within your house adding water, if necessary. It's better to have it too wet than too dry. Keep the bucket covered so as not to allow light through. Darkness is what keeps the leaves white. Within a few days, new growth will begin to appear. Check on your bucket from time to time. Roughly three weeks after stage 6, you should be able to cut your first endive salad. If you started with thick, stocky roots, cut them as you did in step 3 as you may be able to get a second harvest from them. Don't worry if your endives don't hold together tightly in a conical form, the flavor will be the same.
Pack the roots upright in a bucket or pail and fill around them with sand, if you have it, or loose sandy soil if you don't. Regular soil or peat can also be used, but it is difficult to use for filling in the gaps. Cover and store pails in the coolest location available. If you don't have a dark enough room, just use 2 flowerpots, one to hold the roots and another to put on top, to provide you with a dark growing area. Three weeks before you want to enjoy your first endive feast, move the bucket to a 50-60 degree (10-15 celsius) location within your house adding water, if necessary. It's better to have it too wet than too dry. Keep the bucket covered so as not to allow light through. Darkness is what keeps the leaves white. Within a few days, new growth will begin to appear. Check on your bucket from time to time. Roughly three weeks after stage 6, you should be able to cut your first endive salad. If you started with thick, stocky roots, cut them as you did in step 3 as you may be able to get a second harvest from them. Don't worry if your endives don't hold together tightly in a conical form, the flavor will be the same.
What is a seed?
A seed is a small embryonic plant enclosed in a covering called the seed coat, usually with some stored food. It is the product of the ripened ovule of gymnosperm (coniferous) and angiosperm (flowering plants) plants which occurs after fertilization and some growth within the mother plant. The formation of the seed completes the process of reproduction in seed plants (started with the development of flowers and pollination), with the embryo developed from the zygote and the seed coat from the integuments of the ovule.
Which type of seed exist?
Some seeds are closed in a fruit like those of apples or pears, other are open like those from pine cones.
Although there various types of seeds we will be talking about seeds which have two cotyledons and one cotyledon. From this point of view there is a division among plants: those who produce seeds with two cotyledons (like beans or peanuts) or those who produce seeds with one cotyledon (corn, wheat). On the evolution scale we know that plants with one cotyledon appeared more recently than plants with two cotyledons.
What is a seed made of? Which are the parts of the seed?
A seed is a plant in miniature. It has a larger storage area (cotyledon, endosperm) where it keeps its food reserve for germination, it has also an embryo (the miniature plant) and a coat (for protection). Less than 2% of a seed is water. Compare this with 95% which is the quantity of water in a mature herbaceous plant. It is the low water content that protects the seed from frost.
How does germination occur?
When the seeds find the ideal condition the environment, they begin to germinate. First of all their coat is moisten with water and it becomes softer. As soon as the water begins to penetrate the coat and it reaches the embryo, if the seed is viable, the embryo reacts and begins to develop into a plant. First the seed will grow roots that which continue to absorb more water.
Which are the steps of the germination?
Once the coat has been broken, because of the contact with, water the seed will grow roots. A seed is like a sponge, is capable of retaining water. The roots have very fine hairs which help in water absorption. Once the small root is grown then the main stem begins to develop. At this point plants are not capable of photosynthesis and they take their food from the energy stored in their seed (in endosperm and cotyledons). In contact with water the substances (starch, proteins and fats) saved in these storage parts of the seed begin to break down into more simple compounds necessary for the nutrition of the new plant. Once the main stem reaches the surfaces of the soil they, under the influence of light they begin photosynthesis and develop further. They become greener and the leaves will become bigger. The roots continue to absorb water and nutrients from the soil and these are transported to the leaves, where under the influence of light they process this absorbed resources into food necessary for further development of the plant.
What happens with seed once the plant has grown above the ground and begins photosynthesis?
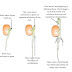
By that time, probably most of the energy reserves are depleted. Certain parts of the seeds may transform into leaves. This is obvious at plants with two cotyledons. This metamorphose ensure that the plants can begin earlier the process of photosynthesis. This first leaves are often named embryonal leaves or false leaves. In the left photos you can see how certains parts a seed metamorphosed.
The photo on the right shows you a healthy seedling of a pumpkin (Curcubita maxima). The cotyledons are transformed into two parallel large leaves (embryonal leaves), capable of photosynthesis, while in the center of the plant the first real leave begins its development.
Why my seedlings are growing long and frail? Why do my seedlings collapse after a while?
This happens when the young seedlings do not have enough light to sustain reliable photosynthesis. If you start a plant from seed in the dark, it will grow using the energy resources. It will grow high looking for light but it will be very fragile. Once the energy source from the seed is depleted the plants will die because they are not able to sustain themselves.
These very youg seedlings of Aster look frail because they dont receive enough light. They are not growing upwards because they twist after the light. The window pane inside a house might be the ideal place to start seeds, but once the seeds germinated need to be moved to a lighter and a colder place.
Do all plants need light in order to germinate?
No. Germination of certain plants is not related to light. Some plants need complete darkness in order to germinate. Sweet pea (Lathyrus odoratus) is this type of plant.
Why certain seeds do not germinate at all?
Certain seeds have a short viability. This means that embryo inside stays alive only for a short while. This is a way in which plants determine that most viable embryos will produce the most viable plants. Certain seeds, like those from cocoa (Theobroma cacao) remain viable only for a few days, while other like those from wild poppies remain viable for decennia. Some other seeds simply do not have an embryo to germinate. This is mostly the case of some modern hybrids of vegetables and decorative plants.
How do the seeds lose their viability?
The seeds lose the capacity to germinate if they become completely dry. This is seeds are kept in lower temperature conditions in the seed banks all over the world.
Why the collected seeds from a certain plant refuse to germinate or take a longer time to germinate?
Plants like any other living organism are determined to conserve their species and survival. This is why certain seeds refuse to germinate immediately. If an acorn would germinate immediately then the winter frost would destroy the young plants. This is the case with the seeds of most deciduous trees. So certain plants purposely delay the germination of their seeds. This is done by providing the seed with a thicker coat, or certain chemicals etc.
Why would plants delay their germination?
Although it may annoying for horticulturist, plants seek the best condition for their flourishing. At the same time plants look after themselves, in the sense that desire to conserve their species. Staggered germination occurs only because of these reasons.
How do plants stagger germination?
One interesting scientific observation is the case of allelopathy (in Greek, "mutual suffering"). Certain plants, like the walnut tree eliminate competition by saturating the neighbouring region with substances than impede germination of its own seeds or even other plants. It is supposed that the roots of the plants release allelochemicals, and this is why farmers cannot grow other crops under walnut trees.
Certain plants produce seeds that can germinate only under specific wavelengths of light. Sunlight is composed of various colours (wavelenghts) Certain seeds desire only red light in order to germinate. In a forest were the canopy is dense, the red light is filtered by the leaves of other plants. A seed that rests on the floor of that forest may wait for years until one of the trees dies, creating thus an open space. When it receives the necessary light will start to germinate. This is mostly the case of evergreen rain forests.
Certain seeds need to be ingested by animals and birds and carried away for miles and miles. In this way plants ensure a wider propagation. However, in order to protect the embryo inside, the coat of the seed is thicker and has to withstand the action of gastric acids from the digestive systems of those animals. Once the seeds are eliminated, and the coat is scarified by the action of these acids, the seed is ready to germinate.
Another unusual requirement for the germination of some seeds is that to be scarified by fire. This treatment applies only to seeds with a thick coat and it is common only among the species living in areas where periodic lightning-fires are part of the balance of the nature. The plants known under the generic name of chapparal are known to germinate under these conditions. After the fire has passed the coat of the seed is scorched and as soon as the first rain comes in they begin to germinate, taking the place of their burnt to death mother plants.
When attempted to grow species of desert flowers, it has been shown that freshly collected seeds germinated the best when they were put for a week in an oven at about 50 degrees Celsius. This actually simulated their native environment. This happens during the summer months in certain desserts. During the winter months, the rains come in and the plants have better conditions for developing and then the seeds would germinate.
The horticulturists often mimic the general conditions of the native geographical areas of the in order to ensure a good germination. Sometimes this proves to be a real challenge. However, in a future article I will discuss about various methods of simulation.